Knowledge
March 1, 2023
Meeting cGMP regulations with innovative single-use technologies
Manufacturers in the pharmaceutical industry are required to comply with cGMP (Current Good Manufacturing Practice). End-to-end solutions based on automated single-use technologies are an auspicious choice for drug substance manufacturers, given their many advantages. Their implementation in the manufacturing process promises efficiency, high product quality, cost reduction, and energy savings.
March 1, 2023
cGMP - everything you need to know
The current Good Manufacturing Practice (cGMP) regulations - originated and checked by the FDA - apply to companies operating in pharmaceutical, biotech, or med tech industries. They require the latest standards in terms of production, manufacturing, and packaging and thereby ensure pharmaceutical quality meaning quality in manufacturing processes and patient safety as the ultimate goal. The detailed requirements, importance of cGMP, and how companies can best meet the cGMP regulations, are discussed in the following.
February 28, 2023
Shipping pharmaceutical liquids and its challenges
The global distribution of pharmaceuticals is complex and demanding, requiring precise handling and storage of sensitive products. With the rise of personalized medicine and the increasing globalization of the industry, the need for safe and efficient shipping and supply chain has never been more important.
February 28, 2023
Reducing product loss & human error in pharmaceutical fermentation
Pharmaceutical manufacturing processes, including pharmaceutical fermentation, are still prone to human error and product loss. The challenges can be overcome by moving to automated processes that eliminate the need for slow and error-prone manual handling. Adopting automated end-to-end solutions based on single-use technologies minimizes, if not eliminates, human error and product loss.
February 17, 2023
History of Fermentation: The journey from brewing to advanced therapies
Fermentation has been with mankind at least since the first settlements in the Middle East about 12,000 years ago. Today, scientists have gained so much knowledge about microorganisms and the fermentation process that pharmaceutical manufacturers are using it for the production of vaccines or bioconjugates like ADCs (antibody-drug conjugates) and the development of advanced therapies.
February 17, 2023
Fermentation in the pharmaceutical industry: A complete guide
Fermentation in the pharmaceutical industry is used to cultivate microorganisms for antibiotics, therapeutic proteins, enzymes and insulin. It typically involves temperature-controlled tanks, also known as fermenters and the correct concentration of nutrients to cultivate the desired organism. Microbial and bacterial fermentation technology and the associated processes open new possibilities and are important building blocks for gene-editing, conjugates and DNA plasmids used in modern vaccine production.
February 14, 2023
Precision Fermentation simply explained
Precision fermentation uses microbial hosts to produce a particular end product. Other terms used for precision fermentation are bacterial and microbial fermentation.
Precision fermentation is used in a variety of ways in both the food industry and pharmaceutical industry to produce biopharmaceuticals. Read more!
February 14, 2023
Microbial Fermentation simply explained
Microbial fermentation is a biochemical process that manages to extract chemical energy from carbohydrates without the oxygen.
This chemical reaction occurs in bacteria, yeasts or even in muscles of humans. Read more details!
February 14, 2023
Differences between microbial fermentation & mammalian cell culture
Microbial fermentation in bacteria, yeast or fungi is, due to its benefits, preferred in the production of smaller biologics. These include peptides, proteins, cytokines, growth factors, plasmid DNA, single-domain antibodies, peptibodies and more. Continue reading...!
February 10, 2023
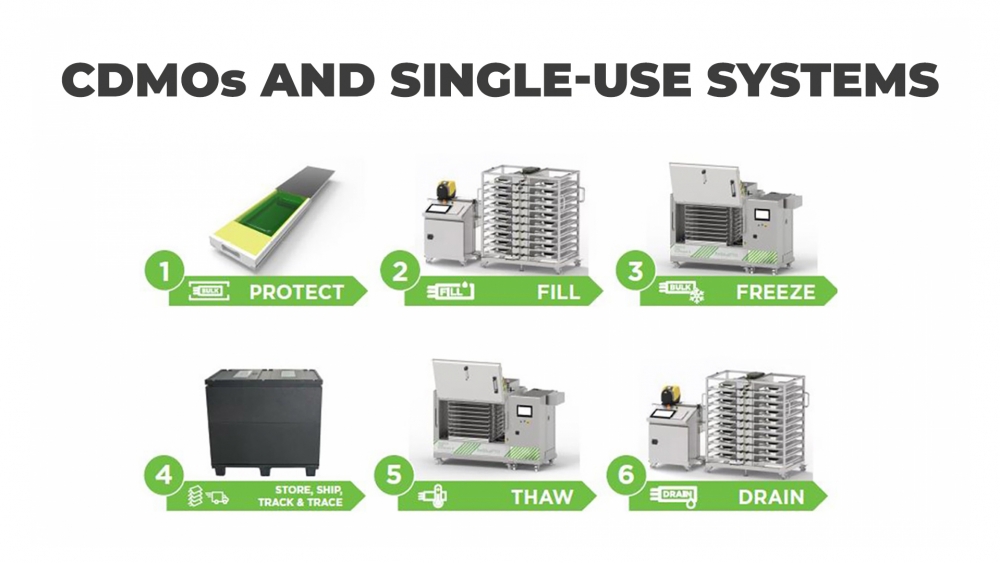
Why CDMOs are increasingly using single-use systems
By combining a number of benefits, single-use technologies that are compliant with cGMP and GAMP seem the perfect choice. For manufacturers of drug substances, they provide a more efficient, cost-effective, and scalable solution than stainless-steel tanks by offering.
February 10, 2023
How to choose a CDMO? 7 Considerations to be made
Choosing the right CDMO or CMO to work with is an important decision. Irrespective of which stage biopharma and pharma companies find themselves in, there are certain things to consider that are valid for any stage throughout your production process.
February 10, 2023
CDMO in Biopharma: Opportunity or risk?
Both, CDMOs and CMOs, are offering major opportunities in biopharma for startups and emerging companies as well as for established players who are approaching the limits of their manufacturing facilities or are generally looking to outsource parts of or their entire commercial production.