Knowledge
April 12, 2022
Extractables and leachables: definitions, differences & facts
Extractables and leachables are contaminants that are causing biopharmaceutical manufacturers headaches, as they can lead to impurities of highly valuable drug products. While extractables and leachables both describe foreign matter that can lead to contamination of highly valuable drug products, it is important to understand their characterization but also the difference between the two.
March 28, 2022
Costs for CAR T-cell therapy - How to deploy cost-efficiency
CAR T-cell treatments are deservedly on the rise, as the effectiveness of CAR T-cell products for formerly hard to treat hematology malignancies has been established in clinical trials. This article will highlight one of the less discussed issues of immunotherapy in cancer treatment: it’s current price tag could hamper the broad accessibility to patients.
March 24, 2022
Storage Density in Biopharma Manufacturing Facilities
Single-use bags with its flat shape is predestined for stacking and offer great solutions to increase storage density on-site but also for shipment. The combination of both, single use bag and RoSS® shells (Robust Storage and Shipping) makes them a cost-effective solution, as they require little storage space. RoSS® shells are available for all sizes and suppliers of single-use bags.
March 23, 2022
How Pharma 4.0 eliminates human errors in pharmaceutical industry
Pharma 4.0 aims at improving risk management. It aims at updating the pharmaceutical industry and ensuring data integrity by incorporating advanced digital formats and enablers into the current Pharmaceutical Quality System (ICH Q10). The final goal is to minimize, if not eradicate, human error.
March 21, 2022
Scalability of single-use drug substance production
The aim was to achieve a freezing process where the last point of freeze always happens at the same time throughout all scales. If successful, the generated freezing curves will look very similar. Temperature ramps have been used to slow down or accelerate the freezing process.
March 9, 2022
Pharma 4.0: Pharmaceutical manufacturing on its journey to modernity
Pharma 4.0 is the vision of digitalized pharmaceutical manufacturing. The implementation of modern digital strategies into the production of pharmaceutical goods promises increasing productivity, easier compliance, enhanced connectivity and improved data monitoring to take appropriate action early. Read more!
March 8, 2022
Single-use tubing assemblies – all parts at a glance
Are you switching your operation to single-use tubing assemblies? In the following, we show you more about the separate components that single-use tubing assemblies and manifolds are made up of and their specific functions.
March 7, 2022
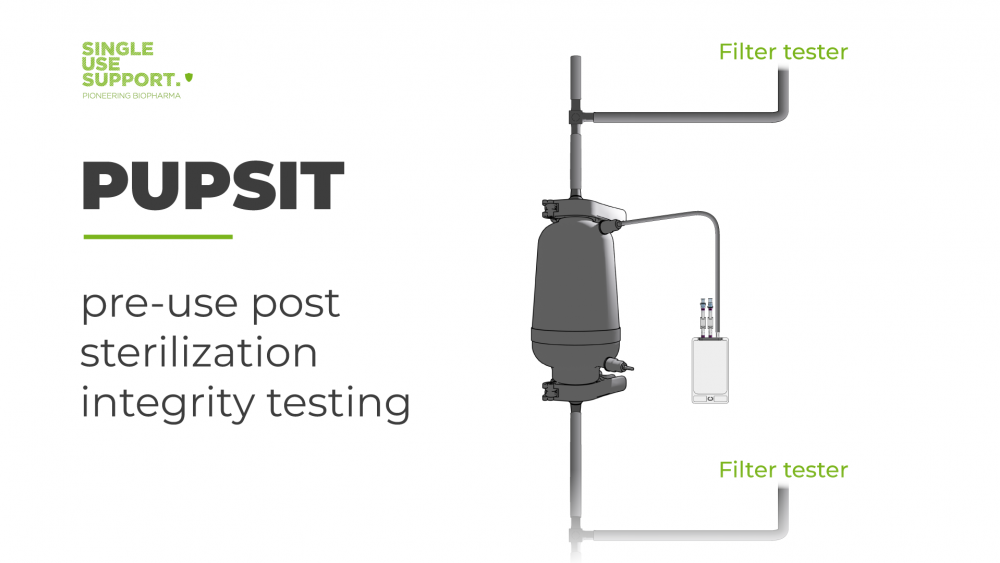
PUPSIT - An Introduction to Pre-use Post Sterilization Integrity Testing
PUPSIT will contribute to simplifying not only integrity testing and validation but manufacturing processes in general, while keeping in line with current good manufacturing processes and any standards and guidelines defined by major authorities such as FDA and EMA. Read all about it!
March 2, 2022
Aseptic bag filling – 5 critical considerations
Aseptic bag filling is a process that is very important to the biopharma industry. This article will give you the basics of aseptic bag filling, highlight the most critical factors of the technique and discuss key considerations pertaining to the commercial use of aseptic bags.
February 15, 2022
Whitepaper: How controlled freezing enables scalability
In a comprehensive study the impact of ice front growth speed on scalability of freezing protein solutions has been evaluated.
February 2, 2022
Inline buffer dilution: an agile system for downstream processes
Inline buffer dilution (IBD) is a method for on demand buffer preparation from buffer concentrates at the point of use. This article will give an overview of the inline buffer dilution process and discuss its benefits over the traditional formulation of buffer solutions.
January 20, 2022
At the forefront of Pharma 4.0
There is no full Pharma 4.0 manufacturing in place yet, but there are first innovations to build on.
Currently numerous manual handling steps in biopharmaceutical manufacturing come along with a high degree of failures in manufacturing facilities deriving from human errors in operative handling.